Trethera Successfully Completes Enrollment of Phase 1 Dose Escalation Trial for Patients with Solid Tumors
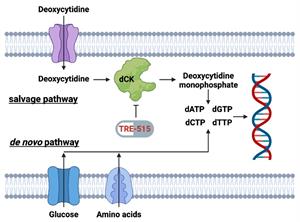
LOS ANGELES, Sept. 10, 2025 (GLOBE NEWSWIRE) -- Trethera Corporation (“Trethera”), a clinical stage biopharmaceutical company developing first-in-class therapies for cancer and autoimmune diseases, announced today that the final patient has enrolled in its Phase 1 dose escalation trial for the treatment of advanced solid tumors. Patients have tolerated escalating doses of Trethera’s lead drug candidate, TRE-515, from 40 mg up to 1,440 mg per day—a 36-fold increase—without any dose-limiting toxicities. TRE-515 inhibits deoxycytidine kinase (dCK), an enzyme critical to the nucleoside salvage pathway that is essential for the growth of abnormal cells in cancer and autoimmune diseases.
“This milestone brings us significantly closer to advancing TRE-515 as the first-to-market dCK inhibitor,” said Dr. Ken Schultz, Trethera’s Chief Executive Officer and Chief Medical Officer. “We remain on track to expand our clinical research in 2026, building on our recent FDA Fast Track designation in prostate cancer.”
Evidence of antitumor activity has been observed even at the lowest dose levels. Multiple patients in the first two cohorts (40 mg and 80 mg) demonstrated clinical benefit, including one who maintained disease control for over 250 days. The trial dosed 33 patients, with several remaining on therapy today, underscoring the favorable safety profile and durability of TRE-515.
The primary endpoints of the Phase 1 trial evaluate safety and tolerability of TRE-515 to determine its maximum tolerated dose. Secondary endpoints include establishing a recommended Phase 2 dose, characterizing pharmacokinetics and pharmacodynamics (PK/PD), and evaluating antitumor activity. The protocol also includes exploratory objectives to measure biomarkers of target engagement. Trethera will present its Phase 1 findings at a major upcoming scientific conference.
The Phase 1 trial was partially supported by a grant from the National Institutes of Health (NIH). During the trial, Trethera’s lead asset, TRE-515, also received:
- Multiple patents with US market exclusivity extended through February 2045,
- FDA Fast Track designation as a combination therapy with radiation to treat prostate cancer,
- $2.3M in new NIH grant funding to study TRE-515 as a prostate cancer therapy with radiation, and
- Two FDA Orphan Drug designations for Acute Disseminated Encephalomyelitis (ADEM) and Optic Neuritis.
For more information regarding the Phase 1 trial, please visit clinicaltrials.gov (NCT05055609).
About Trethera
Trethera is a clinical stage, privately held, biopharmaceutical company dedicated to pioneering the development of novel treatments for autoimmune diseases and cancers. Founded by prominent UCLA scientists, Trethera is led by experienced management and board members. Trethera's innovative approach to targeting nucleotide metabolism led to the development of TRE-515, an orally administered capsule. TRE-515 is a first-in-class clinical stage drug that inhibits deoxycytidine kinase (dCK), the rate-limiting enzyme in the nucleoside salvage pathway, one of two biosynthetic pathways that generate DNA precursors. It is believed that some forms of cancer may be preferentially dependent on the salvage pathway to support tumor growth, and certain autoimmune diseases might also respond to TRE-515 treatment. The FDA has designated TRE-515 a Fast Track drug for prostate cancer and an Orphan Drug for two autoimmune neurologic diseases. Trethera is developing TRE-515 for use as a monotherapy or in combination to precisely target a metabolic vulnerability of cancer or autoimmune diseases that will transform outcomes for patients.
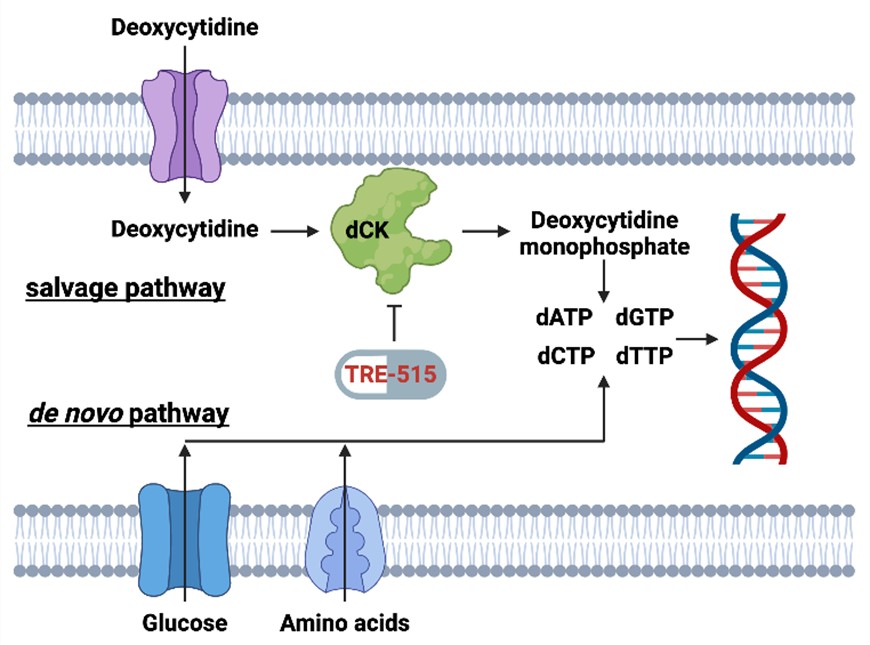
Figure 1: Biochemical pathways for the supply of deoxyribonucleoside triphosphate pools. The salvage pathway becomes upregulated during autoimmune diseases and cancer. TRE-515 blocks the enzyme deoxycytidine kinase (dCK) in the deoxyribonucleoside salvage pathway.
For more information, please visit us at trethera.com or e-mail Investor Relations at ir@trethera.com. You can also follow Trethera on Facebook and LinkedIn.
Note on Forward-Looking Statements
All statements other than statements of historical facts included in this press release that address activities, events or developments that Trethera believes or anticipates will or may occur in the future are “forward-looking statements,” which may often, but not always, be identified by the use of such words as "may," "might," "will," "will likely result," "would," "should," "estimate," "plan," "project," "forecast," "intend," "expect," "anticipate," "believe," "seek," "continue," "target" or the negative of such terms or other similar expressions. Although Trethera has a reasonable basis for the forward-looking statements contained herein, Trethera cautions that such statements are based on current expectations about future events and are subject to risks, uncertainties and factors relating to medical and scientific research, all of which are difficult to predict and many of which are beyond Trethera’s control, that may cause actual results to differ materially from those expressed or implied by the forward-looking statements in this press release. These potential risks and uncertainties include, without limitation: the extent to which development of any novel cancer therapies or therapies for autoimmune diseases succeeds; whether Trethera would obtain the necessary regulatory approvals to commence human trials or commercialize TRE-515 or any novel therapies resulting from such research; Trethera successfully implementing its growth strategy, including that relating to its disease therapies; the effects of the global Covid-19 pandemic; changes in economic conditions; competition; and risks and uncertainties applicable to the business of Trethera. The statements in this press release speak only as of the date hereof and Trethera does not undertake any obligation to update, amend or clarify these forward-looking statements whether as a result of new information, future events or otherwise. The Company intends that all forward-looking statements be subject to the safe-harbor provisions of the Private Securities Litigation Reform Act of 1995.
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/f99e7bcd-4263-4aab-b8d2-1638ab76f2bd

Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.